 Table of Contents for Caveman Chemistry: 28 Projects, from the Creation of Fire to the Production of Plastics Table of Contents for Caveman Chemistry: 28 Projects, from the Creation of Fire to the Production of Plastics | ||
|---|---|---|
| <<< Previous | Chapter 15. al-Razi (Stoichiometry) | Next >>> |
 Table of Contents for Caveman Chemistry: 28 Projects, from the Creation of Fire to the Production of Plastics Table of Contents for Caveman Chemistry: 28 Projects, from the Creation of Fire to the Production of Plastics | ||
|---|---|---|
| <<< Previous | Chapter 15. al-Razi (Stoichiometry) | Next >>> |
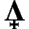
The chapter began with Muhammad bin Zakarīyā al-Rāzī's favorite recipe for making sodium hydroxide (caustic soda, lye) from sodium carbonate (al-Qilī, alkali, soda) and calcium hydroxide (slaked lime). This classic metathesis reaction ought to be familiar to anyone who managed to stay awake during Chapter 7. This recipe, one part soda with one part lime, brings up one of the most fundamental problems in all of chemistry; what, exactly, is a part?
In Chapter 7 I told you that the little numbers in front of each substance in a balanced chemical equation are called stoichiometric coefficients and that their unit is the mole. A mole is just a number like a dozen or a hundred or a million, but much bigger. Just as the weight of a dozen lemons would be expected to be different from the weight of a dozen tea-bags, the weight of a mole depends on what it is you're talking about.
The truth of the matter is that if you have a grip on Unit Factor Analysis , three new unit factors will allow you to solve any stoichiometric problem. And these three unit factors share a common unit, (grams/mole). The first of these is the atomic weight, the weight of one mole of atoms; the second is the formula weight, the weight of a mole of formula; similarly the molecular weight is the weight of a mole of molecules. Formula and molecular weights come from adding up the atomic weights for the elements in an empirical or molecular formula, respectively, and atomic weights may be found either in a periodic table or in Appendix E.
So let's jump right in with a problem from al-Rāzī:
A: To answer any stoichiometry problem we must begin with a balanced equation:
From the balanced equation we get a mole ratio, in this case, for every mole of soda (sodium carbonate), one mole of slaked lime (calcium hydroxide) is consumed; one mole of limestone (calcium carbonate) and two moles of caustic soda (sodium hydroxide) are produced. Note that relative number of moles is given by the stoichiometric coefficient, the little number in from of each reactant or product in a balanced chemical equation. When there is no number in front of a reactant or product, it is assumed to be one. The unit factor required for this particular problem is (1 mole soda/1 mole slaked lime) or (1 mole slaked lime/1 mole soda), depending on the units we need in the top and bottom of the factor.
For the formula weights, we just add up the atomic weights; for soda, we get (2×23 + 12 + 3×16) = (106 g/mole); for slaked lime we get (40 + 2×16 + 2×1) = (74 g/mole).
That is, each part of slaked lime reacts with 1.43 parts of soda. So 1 part lime to 1 part soda may be almost 50% off, but it's not half bad for a recipe from the tenth century.
Remember that a string of unit factors can be continued from one line to the next and that a second "=" sign begins a second equation which is equal to the first. You could easily have worked this problem in Chapter 3 if you had been given the unit factors in the statement of the problem. The only new things in this chapter are the formula weights and the mole ratios.
A: So far, I have used the terms soda ash and washing soda interchangeably. When either one dissolves in water it immediately dissociates into sodium ions and carbonate ions. Soda dissolved in water has no "memory" of where it came from, so chemically speaking, anhydrous (without water) soda and washing soda can be used interchangeably. Nevertheless, they are not exactly the same substances; in particular, their formula weights differ and this is relevant to the problem of how much to weigh out to achieve a balanced reaction. Similarly, as soon as quicklime hits the water, it slakes and the resulting slaked lime is identical to the slaked lime of the previous problem. But the weight of lime we should use depends very much on whether we are using quicklime or slaked lime. When weighing out chemicals, it is vital to know whether the material is hydrous or anhydrous because you must account for the weight of any water present.
The formula weights of washing soda and quicklime are 286 g/mole and 56 g/mole, respectively.
About 5 parts of washing soda are required to react with each part of quicklime.
A: There are two wrinkles in this one; the mole ratio is no longer 1:1. The word excess tells us that there is "more than enough soda". We assume that all of the quicklime is consumed and that there is leftover soda when the reaction is done. The reactant that runs out first, in this case quicklime, is known as the limiting reagent, the one which is not in excess. For now, the word excess tells you that you only need to deal with the other reactant.
You may be disturbed by the notion that you can make 146 g of caustic soda from only 100 g of quicklime. Is the Law of Conservation of Mass violated here? Not at all! If you calculate the number of grams of soda reacting with that 100 grams of quicklime, you will see that the weight of the soda plus the weight of the quicklime is exactly the same as the weight of the caustic soda plus the weight of the chalk plus the weight of the water produced.
A: This would be a very easy experiment to perform. Just put a weighed amount of washing soda into a hot oven (above 100°C) for a few hours and weigh it again when it is done. Of course, you would substitute your actual weight of washing soda for the "100 grams" in the stated problem.
The formula weights of washing soda and anhydrous soda are 286 g/mole and 106 g/mole, respectively.
Your calculated weight of product, in this case 37 g of anhydrous soda, is called the theoretical yield. It can be compared to your actual weight, the experimental yield. When the theoretical and experimental yields are close to one another, you have a happy, comfortable feeling that you understand what's going on and that you are getting the most from your reaction. If they are different from one another, you have an uncomfortable feeling that you don't understand what's going on, and that you are not getting everything from your reaction that you might. Think about some situations that might result in the theoretical yield being higher or lower than the experimental yield. Then try it for yourself.
A: Now, you were supposed to have weighed your pot before and after firing, and so at this point you can see for yourself whether Athanor was feeding you a line.
There are several conditions assumed by the question itself. The calculation assumes that we started with pure, dry kaolinite, a condition that may have been only approximated in your clay body. It assumes that Equation 5-1 is the only reaction taking place and that all of the kaolinite was converted, that is, no un-reacted kaolinite remains in your fired pot. And it assumes that nothing stuck to or flaked off of your pot. Substituting the weight of your un-fired pot for the "100 grams" of the problem, calculate a theoretical yield and compare it to the experimental yield. Of course, you have to calculate the theoretical weights of mullite and silica separately and then add them to get the theoretical weight of your pot. If your theoretical and experimental yields are close to one another, it doesn't necessarily mean that everything Athanor told you was true, it just means that your evidence supports his assertions. Scientific theories are never proven once and for all; but the more evidence supports a theory, the more confident we become that it is essentially correct.
 | Material Safety |
|---|---|
If you're like most people you find the information in an MSDS rather daunting. It's full of all kinds of technical information suited to occupational safety. Wouldn't it be great if there were some kind of simple, easily understood safety rating system so that you would know whether a chemical was really, really dangerous, or just dangerous in the way that rocks and sticks and dirt are dangerous? Well, there is such a system. You've probably seen symbols like those in Figure 15-1 which provide information on hazardous materials, but most people don't have a clue what they mean. The National Fire Protection Agency[1] developed the NFPA diamond to give fire fighters information on hazardous chemicals at a glance. The number in the left-most (blue) quadrant is the health rating, that in the upper-most quadrant (red) is the flammability rating, that in the right-most (yellow) quadrant is the reactivity rating, and the number in the lowest (white) quadrant gives special hazards, for example, OX for oxidizer or W for exceptional reactivity with water. The numerical ratings run from 0 (not very dangerous) to 4 (very dangerous). Most MSDS's give the NFPA ratings, either as a diamond graphic or as text (NFPA H 1, F 0, R 0, OX). Let's look at the NFPA diamonds (Figure 15-1) for four materials which will play a large role throughout the remaining chapters. We've already been introduced to ethanol in Chapter 4 and will see more of it in Chapter 16. As a poison, ethanol is fair to middling and gets a "2" in the health quadrant. In the flammability quadrant ethanol gets a "3;" it's quite flammable, though not as flammable as something like propane. In the reactivity quadrant ethanol gets a "0;" it's not particularly reactive with common materials and is not explosive. The special quadrant is blank, as ethanol isn't an oxidant and it isn't particularly reactive with water. Ethanol's NFPA symbol tells a firefighter that flammability is its most important property in an emergency situation. We met caustic soda, sodium hydroxide, in Chapter 14 as an excellent alkali for making paper. Sold in the grocery store as "lye," it's a common ingredient in household drain openers. Caustic soda will emerge as an important player in Chapter 19. In the present chapter we've explored the stoichiometry for making caustic soda from soda and lime. Caustic soda is fairly toxic by ingestion, but the fact that it eats skin bumps its health rating up to a "3." Caustic soda will not burn, so it gets a "0" for flammability. It is reactive with some other materials, notably aluminum, and so it gets a "1" for reactivity. While it gets hot on contact with water, this is not enough to get it a notice in the special quadrant. Thus caustic soda's NFPA symbol tells the firefighter that exposure to the material should be avoided, but that it is neither flammable nor explosive. Ordinary laundry bleach is a solution of sodium hypochlorite in water. It is roughly as poisonous as caustic soda and gets a "3" for health. It's not flammable so it gets a "0" for flammability. It's not particularly reactive so it gets a "0" for reactivity. It is an oxidizer, meaning that it can substitute for oxygen in a fire, a fact to which fire fighters are alerted in the special quadrant. We'll meet nitroglycerin in Chapter 27, though you probably know it already as a powerful explosive. While it's moderately toxic and flammable, its most important property from a firefighting point of view is that it will blow you to kingdom come. That gets it the highest rating, a "4," in the reactivity quadrant. The diamond was designed for fire fighters and the NFPA doesn't encourage its use for other purposes. The diamond, for example, doesn't alert people to chronic toxic effects or carcinogenicity. Thus it's less relevant to hazards associated with long-term occupational exposure than it is to acute exposure under emergency situations. At least two other rating systems have emerged to deal with occupational and laboratory situations: the HMIS rating of the National Paint and Coatings Association[2] and the Saf-T-Data rating of the J. T. Baker chemical company.[3] Like the NFPA diamond, both systems use a numerical rating from 0 (not hazardous) to 4 (very hazardous) in four or five categories akin to those of the diamond. Unlike the diamond, however, these systems factor the effects of chronic exposure into the health rating and so the numerical ratings may differ somewhat from one system to another. Either the HMIS or Saf-T-Data systems would appear to be preferable to the NFPA diamond for laboratory use, but neither has been adopted as widely as the diamond. Since the diamond is nearly universal and since chemical exposure for cavemen is likely to be acute rather than chronic, we'll concern ourselves primarily with the diamond as a shorthand for chemical hazards. Still, it's only a shorthand and not a substitute for the full MSDS. You aren't going to get away without a material safety assignment, even though there are no materials involved in this project. If you don't have them already, search for MSDS's using the keyword "NFPA." Draw the NFPA diamonds for slaked lime (CAS 1305-62-0), propane (CAS 74-98-6), and silica (CAS 14808-60-7) in the material safety section of your write-up for this project. |
 | Research and Development |
|---|---|
So there you are, studying for a test, and you wonder what will be on it.
|
| [1] | For more information on the NFPA diamond, see Reference [35]. |
| [2] | For more information on the HMIS system, see Reference [37]. |
| [3] | For more information on the Saf-T-Data system, see Reference [31]. |
| <<< Previous | Home | Next >>> |
| al-Razi (Stoichiometry) | Up |  |